Clinical Trials
Clinical Trials
CIRT

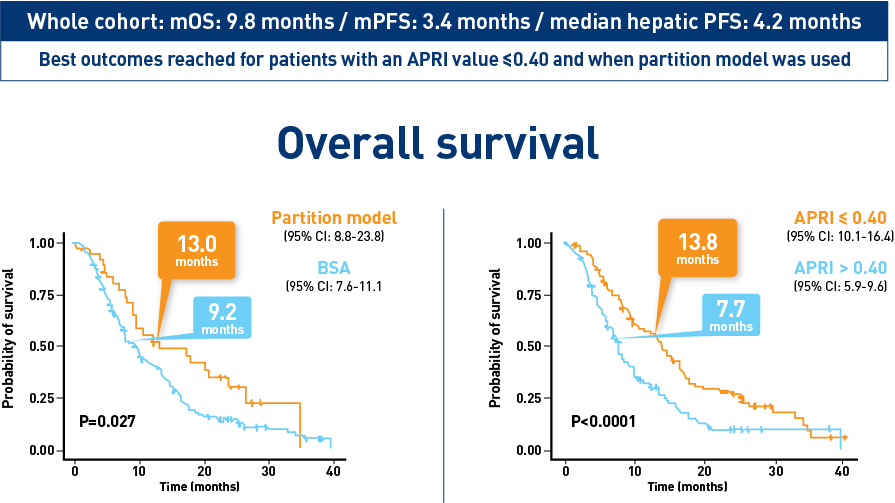
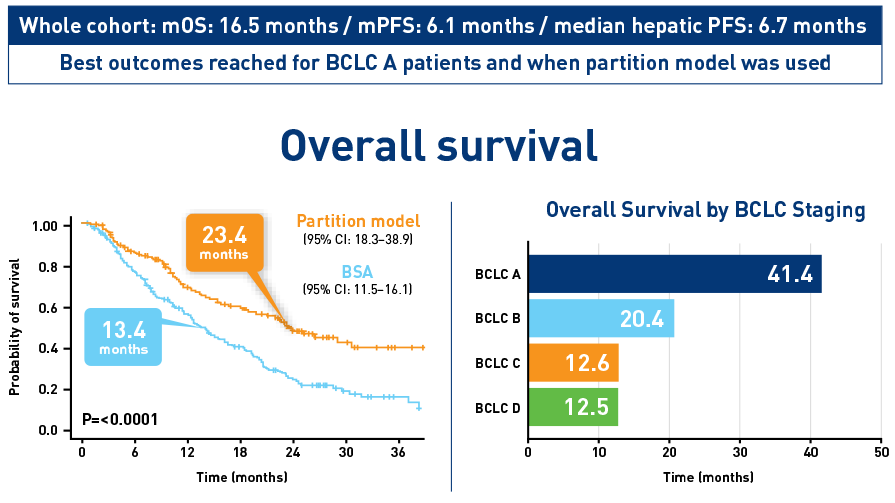
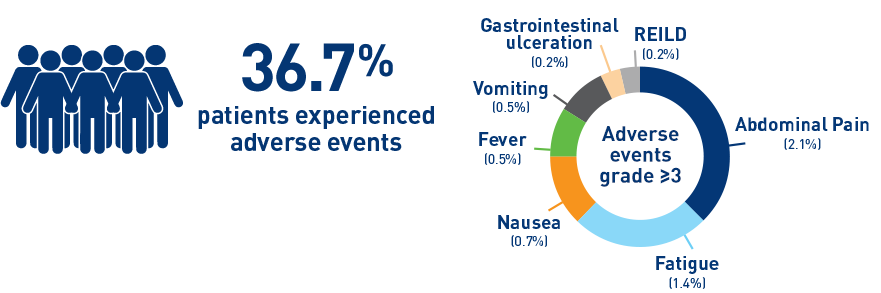
Results from the CIRT study confirm safety and effectiveness of SIRT with Y-90 resin microspheres within the real-world setting and confirm findings from the earlier studies1-3
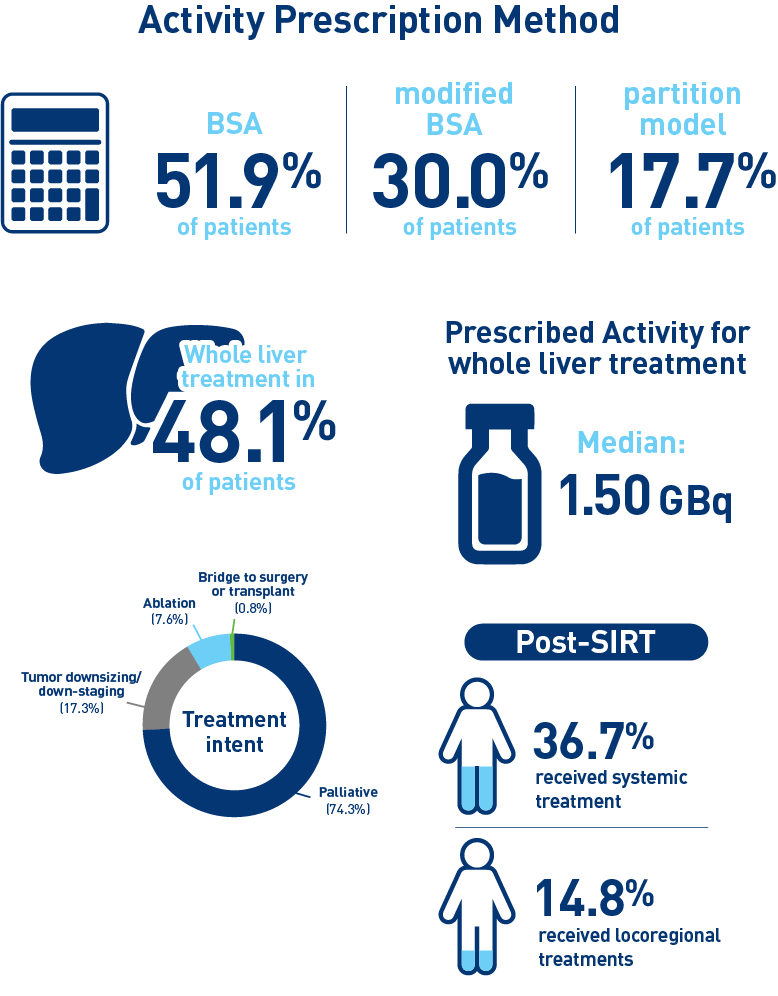
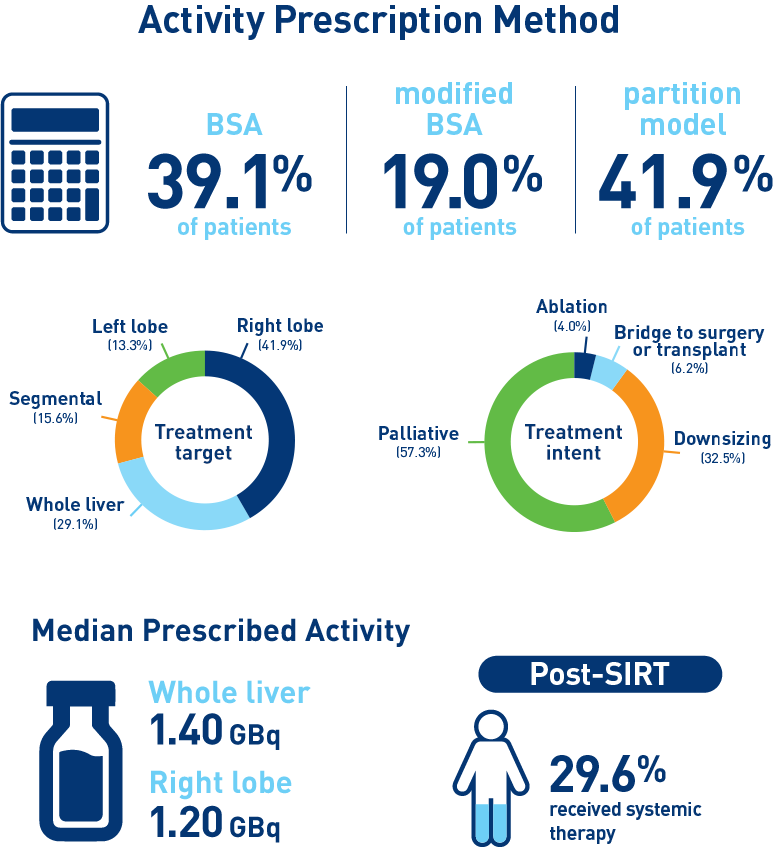
Results from the mCRC cohort1,2

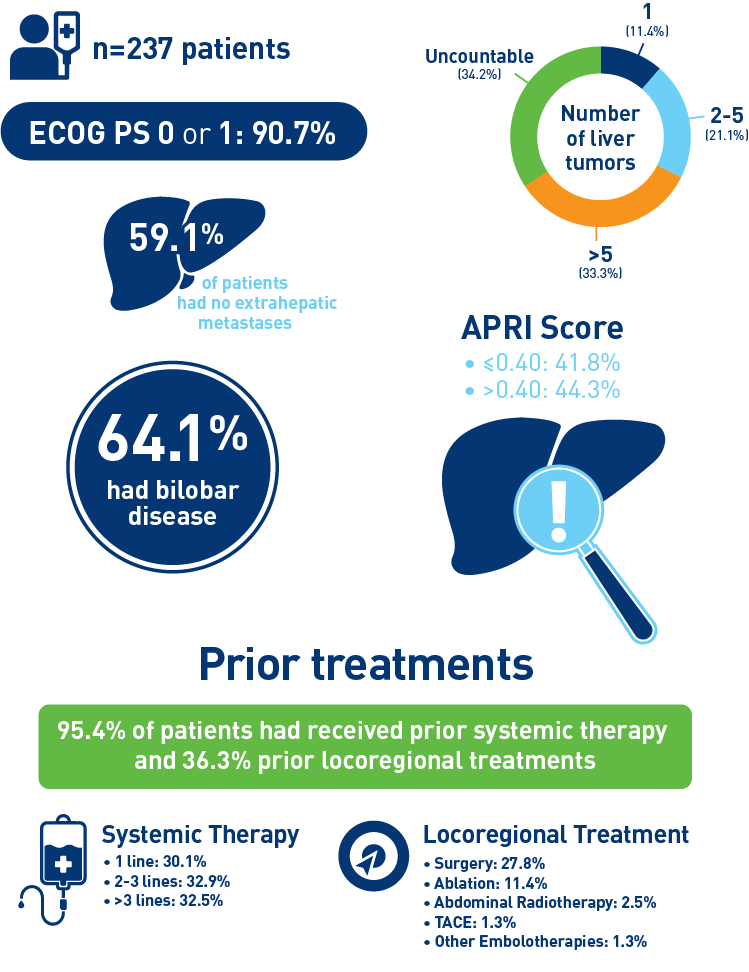
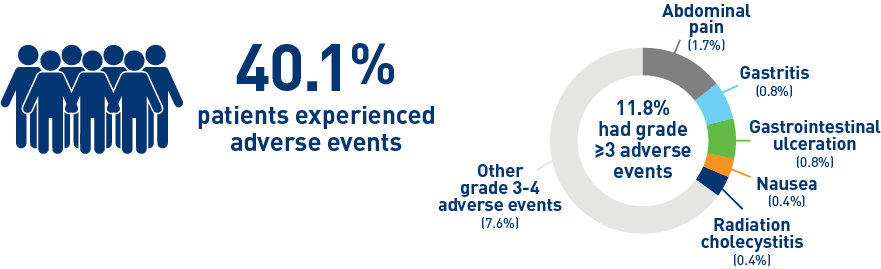
Results from the HCC cohort1,3
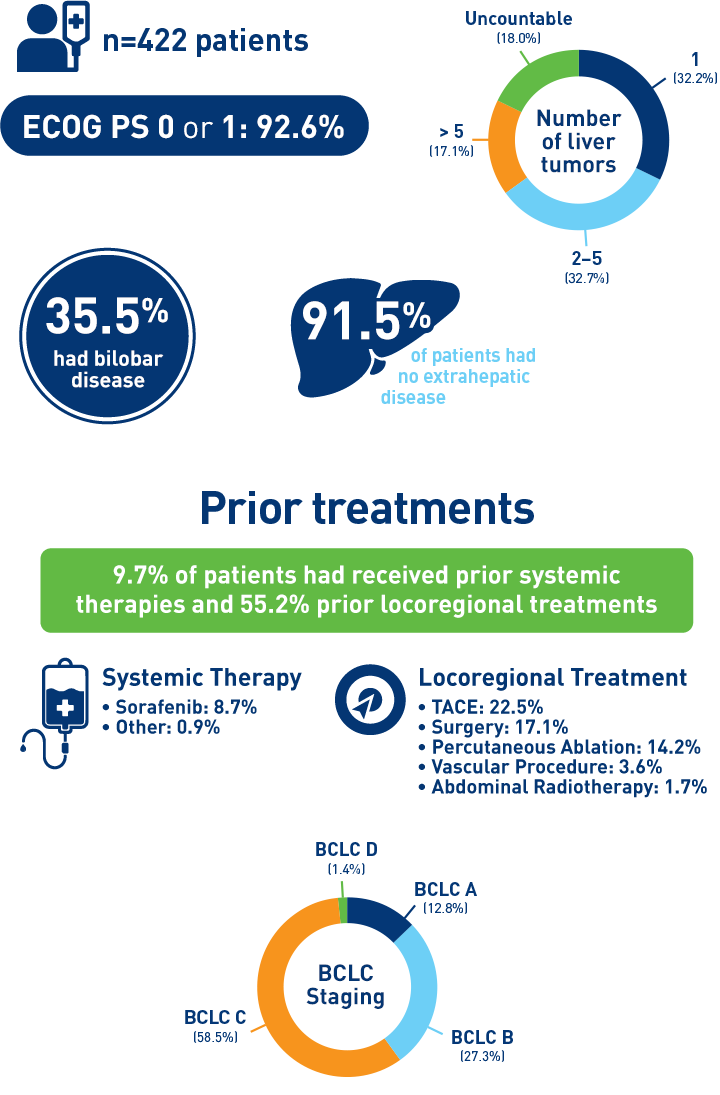
Abbreviations:
HCC: Hepatocellular carcinoma ; mCRC: metastatic colorectal cancer ; ECOG PS: Eastern Cooperative Oncology Group - Performance Status ; APRI: AST to Platelet Ratio Index ; TACE: Transarterial chemoembolization ; BSA : Body surface area ; OS: Overall survival ; PFS: Progression-free survival ; REILD: Radioembolization-Induced Liver Disease
References:
1. Helmberger T et al. Cardiovasc Intervent Radiol 2021; 44: 21–35. 2. Schaefer N et al. Clin Colorectal Cancer 2022; doi: 10.1016/j.clcc.2022.09.002. 3. Kolligs et al. JHEP Reports 2022; 5:100633. doi: 10.1016/j.jhepr.2022.100633
SIR-Spheres® Y-90 resin microspheres are indicated for the treatment of:
- Unresectable hepatocellular carcinoma (HCC), or
- Unresectable metastatic liver tumors from primary colorectal cancer (mCRC) in patients refractory to or intolerant of chemotherapy, or
- Unresectable intrahepatic cholangiocarcinoma (iCCA), or
- Hepatic metastases by neuroendocrine tumours (mNET), or
- Other liver metastases