Clinical Trials
DOORwaY90 Study
>




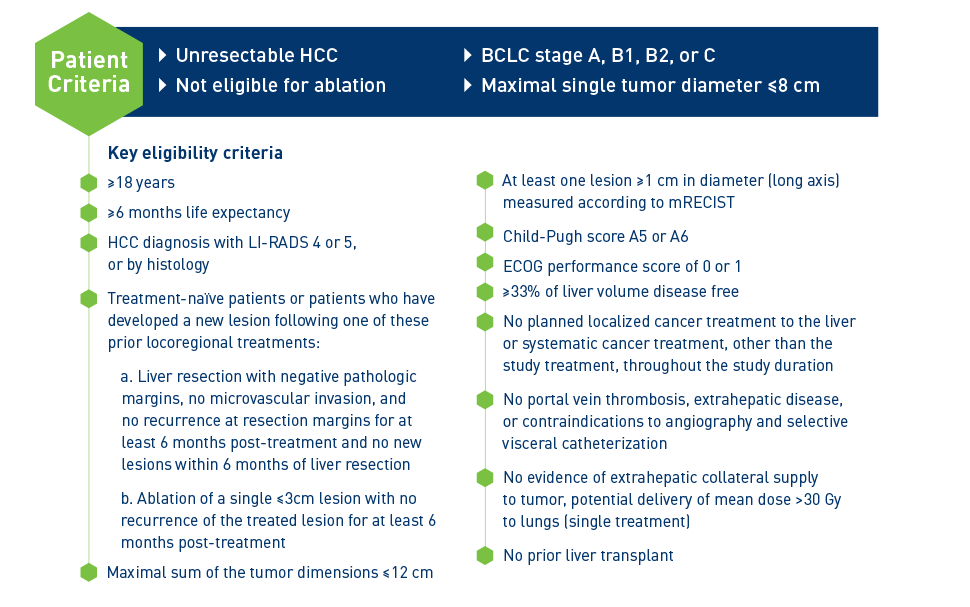
BCLC, Barcelona Clinic Liver Cancer, ECOG, Easter Cooperative Oncology Group; EQ-5D-5L, EuroQoL 5-Dimensions 5-Level; FACT-Hep, Functional Assessment of Cancer Therapy-Hepatobiliary; HCC, hepatocellular carcinoma; LI-RADS, Liver Imaging Reporting And Data System; mRECIST, modified Response Evaluation Criteria in Solid Tumors; QoL, quality of life; SIRT, selective internal radiation therapy.
SIR-Spheres® Y-90 resin microspheres are indicated for the treatment of:
- Unresectable hepatocellular carcinoma (HCC), or
- Unresectable metastatic liver tumors from primary colorectal cancer (mCRC) in patients refractory to or intolerant of chemotherapy, or
- Unresectable intrahepatic cholangiocarcinoma (iCCA), or
- Hepatic metastases by neuroendocrine tumours (mNET), or
- Other liver metastases