Clinical Trials
Clinical Trials
CIRT

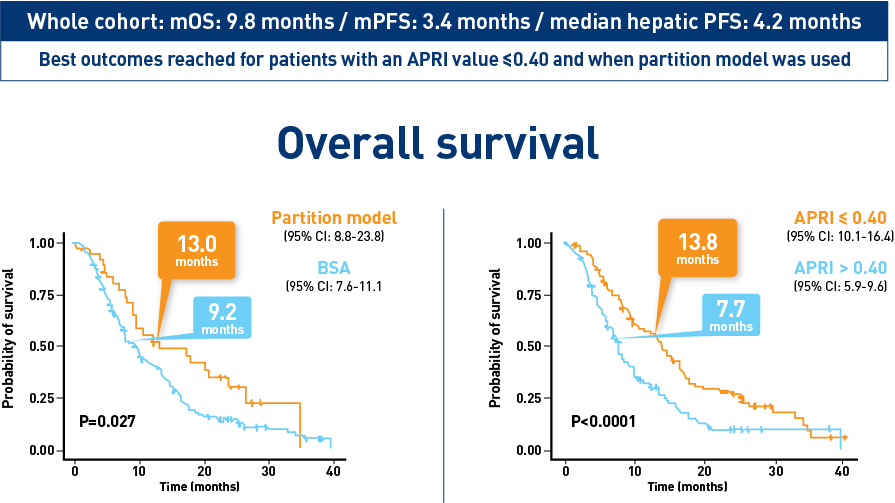
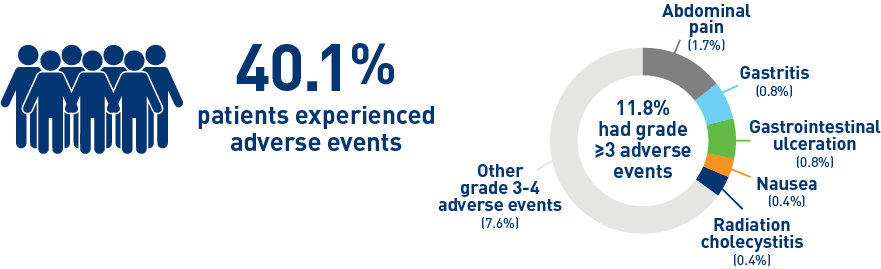
Results from the CIRT study confirm safety and effectiveness of SIRT with Y-90 resin microspheres within the real-world setting and confirm findings from the earlier studies1-3
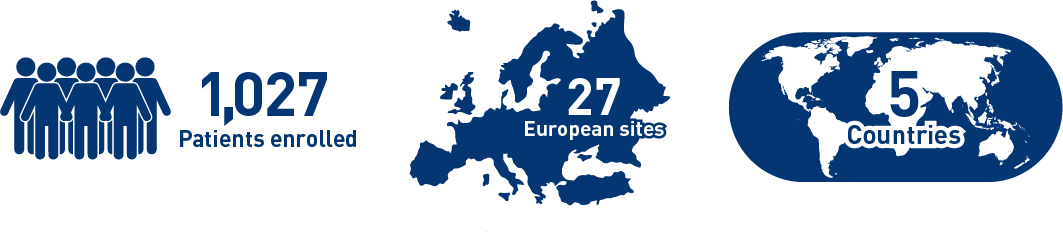
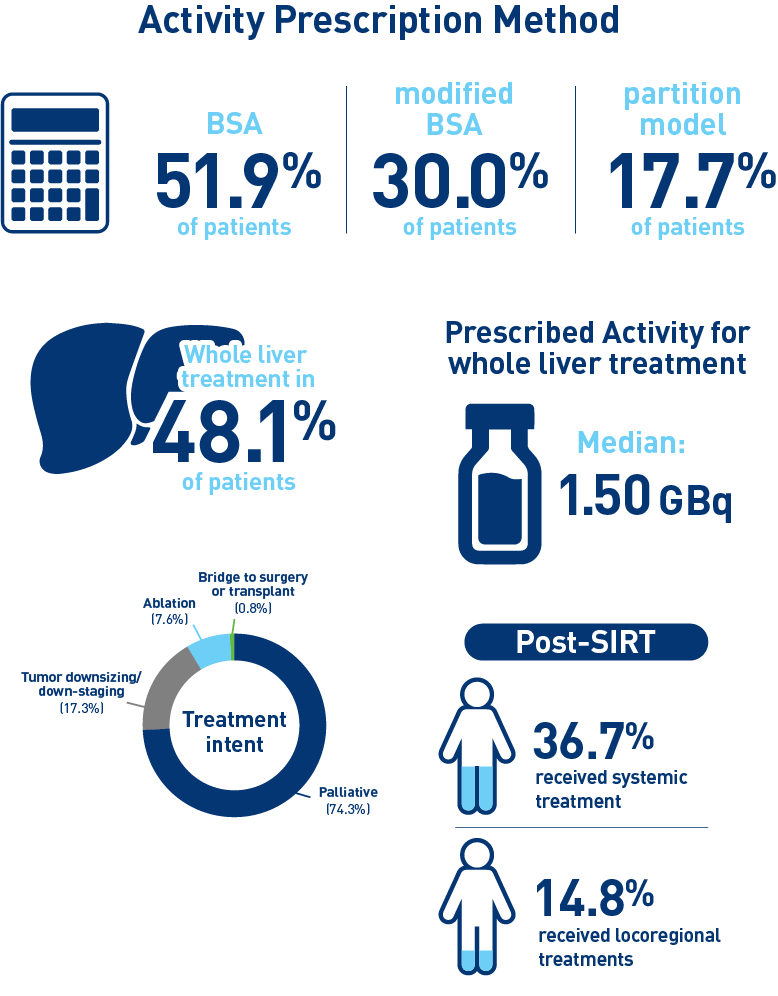
Results from the mCRC cohort1,2

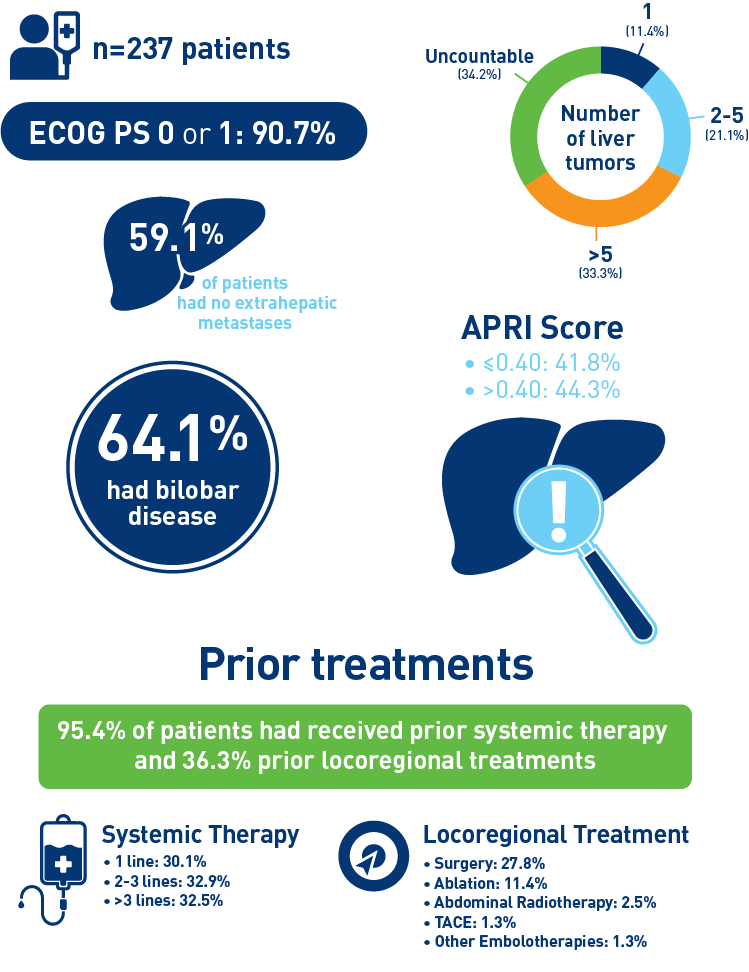
Abbreviations:
HCC: Hepatocellular carcinoma ; mCRC: metastatic colorectal cancer ; ECOG PS: Eastern Cooperative Oncology Group - Performance Status ; APRI: AST to Platelet Ratio Index ; TACE: Transarterial chemoembolization ; BSA : Body surface area ; OS: Overall survival ; PFS: Progression-free survival ; REILD: Radioembolization-Induced Liver Disease
References:
1. Helmberger T et al. Cardiovasc Intervent Radiol 2021; 44: 21–35. 2. Schaefer N et al. Clin Colorectal Cancer 2022; doi: 10.1016/j.clcc.2022.09.002. 3. Kolligs et al. JHEP Reports 2022; 5:100633. doi: 10.1016/j.jhepr.2022.100633
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician Indications for Use: SIR-Spheres® Y-90 resin microspheres are indicated for the local tumor control of unresectable hepatocellular carcinoma (HCC) in patients with no macrovascular invasion, Child Pugh-A cirrhosis, well-compensated liver function, and good performance status. They are also indicated for the treatment of unresectable metastatic liver tumors from primary colorectal cancer with adjuvant intra-hepatic artery chemotherapy (IHAC) of FUDR (Floxuridine). Side Effects: Common side effects are fever, transient decrease of hemoglobin, mild to moderate abnormality of liver function tests, abdominal pain, nausea, vomiting, and diarrhea. Potential serious effects due to exposure to high radiation include acute pancreatitis, radiation pneumonitis, acute gastritis, acute cholecystitis and radioembolization induced liver disease (REILD). Consult the Instructions for Use for a complete listing of indications, contraindications, side effects, warnings, and precautions.