Clinical Trials
DOORwaY90 Study
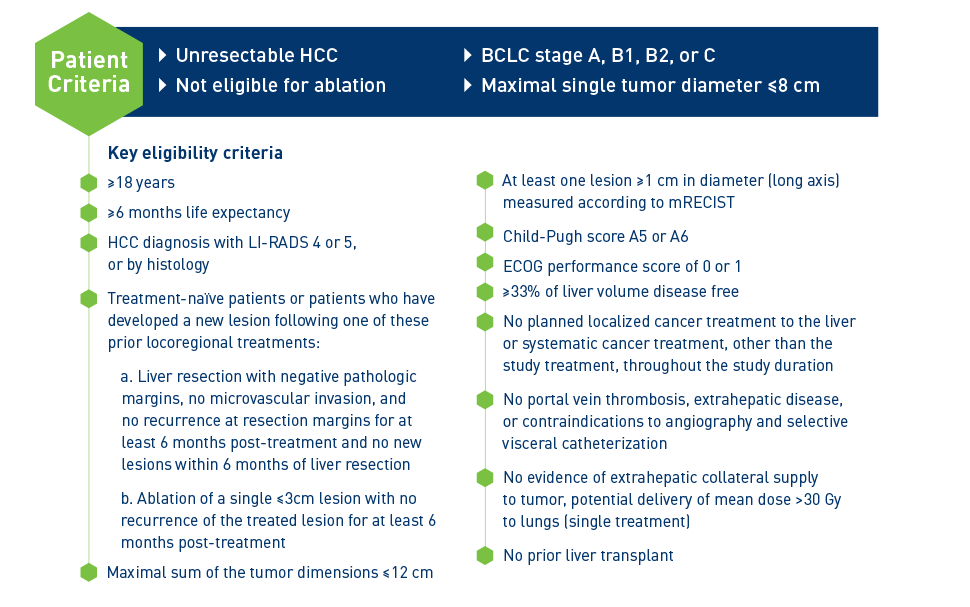
BCLC, Barcelona Clinic Liver Cancer, ECOG, Easter Cooperative Oncology Group; EQ-5D-5L, EuroQoL 5-Dimensions 5-Level; FACT-Hep, Functional Assessment of Cancer Therapy-Hepatobiliary; HCC, hepatocellular carcinoma; LI-RADS, Liver Imaging Reporting And Data System; mRECIST, modified Response Evaluation Criteria in Solid Tumors; QoL, quality of life; SIRT, selective internal radiation therapy.