Clinical Trials
Clinical Trials
RESIN


Real-world data from RESiN support evidence on the efficacy and safety of SIRT with
SIR-Spheres® Y-90 resin microspheres2-4
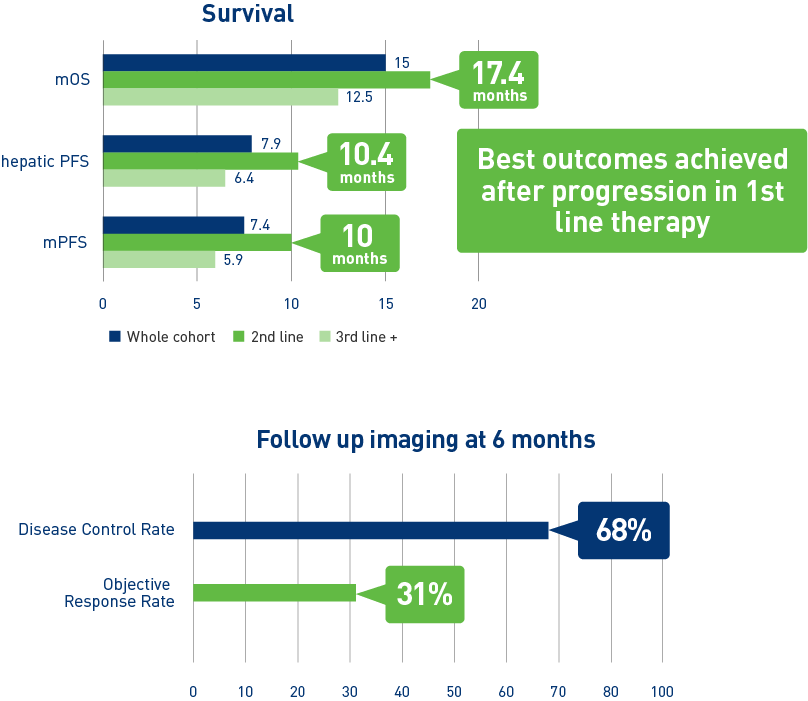
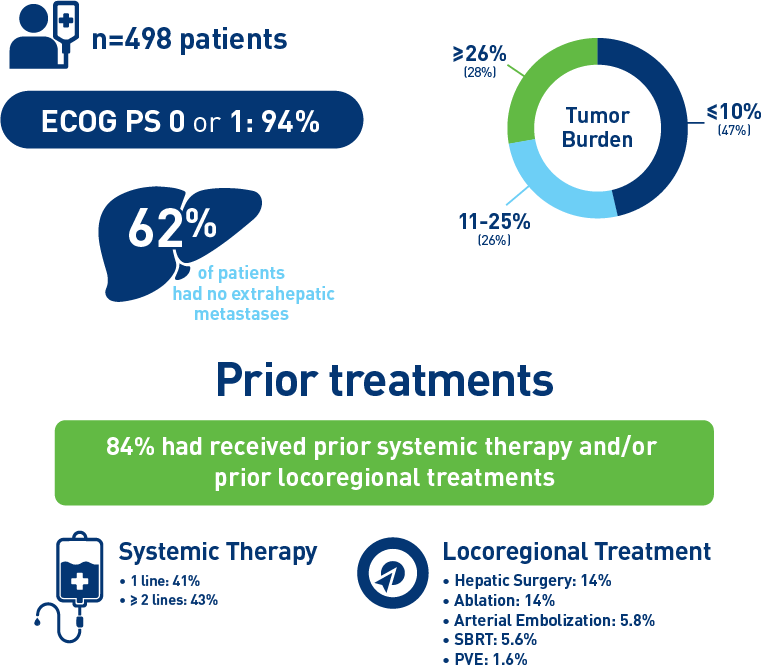
Results from the mCRC cohort3,4
Abbreviations:
HCC: Hepatocellular carcinoma ; mCRC: metastatic colorectal cancer ; ECOG PS: Eastern Cooperative Oncology Group - Performance Status ; SBRT: Stereotactic Body Radiation Therapy ; PVE: Portal vein embolization ; BSA : Body surface area ; OS: Overall survival ; PFS: Progression-free survival ; BCLC: Barcelona clinic liver cancer ;
References:
- Sirtex Press Release Aug 2020. https://appliedradiationoncology.com/articles/sirtex-completes-u-s-resin-registry-enrollment. Last access: Aug 12th 2021. 2. Frantz S et al. J Vasc Interv Radiol 2021; 32: 845–852. 3. Brown D et al. J Gastrointest Oncol 2021; 12(2):639-657. 4. Emmons et al. Radiol 2022; doi: 10.1148/radiol.220387.