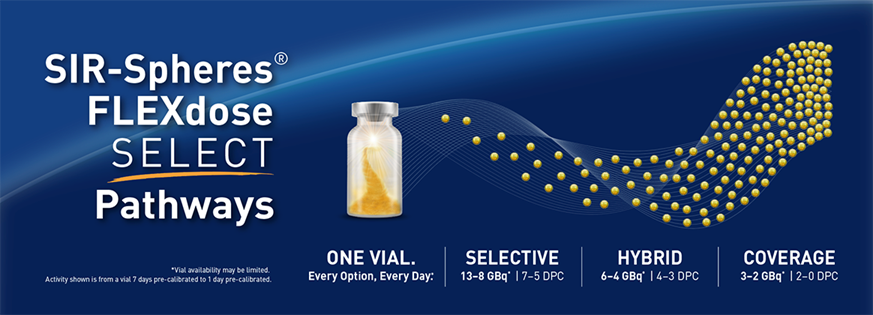
FLEXdose SELECT Pathways
The FLEXdose SELECT Pathways; offering up to 7* delivery options every day of the week, aims to enhance the precision, effectiveness, and convenience of SIR-Spheres Y-90 resin microsphere therapy.
SIR-Spheres® FLEXdose SELECT Pathways offers significant value to both patients and physicians in the treatment of metastatic colorectal cancer (mCRC) of the liver and hepatocelluar carcinoma (HCC) using SIR-Spheres Y-90 resin microspheres.
The Treating Physician:
- Ease of Use
Streamlined ordering and delivery process for seamless integration into your clinical workflow. - Convenience
On-demand access to personalized Y-90 resin doses tailored to your patient's needs.
Flexible scheduling options to accommodate your treatment timelines. - Flexibility
Customizable dose ranges to precisely match your prescribed treatment plan.
Adaptable to various delivery techniques preferred by your clinical team. - Control
Empowers your treatment team with full control of dosing and administration. - Adaptability
It accommodates various delivery techniques preferred by different clinical teams.
For Patients:
- Customized Treatment
Patients receive a treatment plan precisely tailored to their specific tumor burden and characteristics. - Precise Activity Delivered
Optimal personalized dosing delivers the exact required radioactive activity. - Optimal Tumor Coverage
Careful selection of microsphere volume ensures comprehensive tumor coverage and maximized absorbed dose.
One Vial. Every Option, Every Day.
*Vial availability may be limited.




